Clinical Study Conducted at Medical University of South Carolina Finds Synchrony Medical’s LibAirty™ Airway Clearance System Highly Effective for Chronic Lung Disease Patients
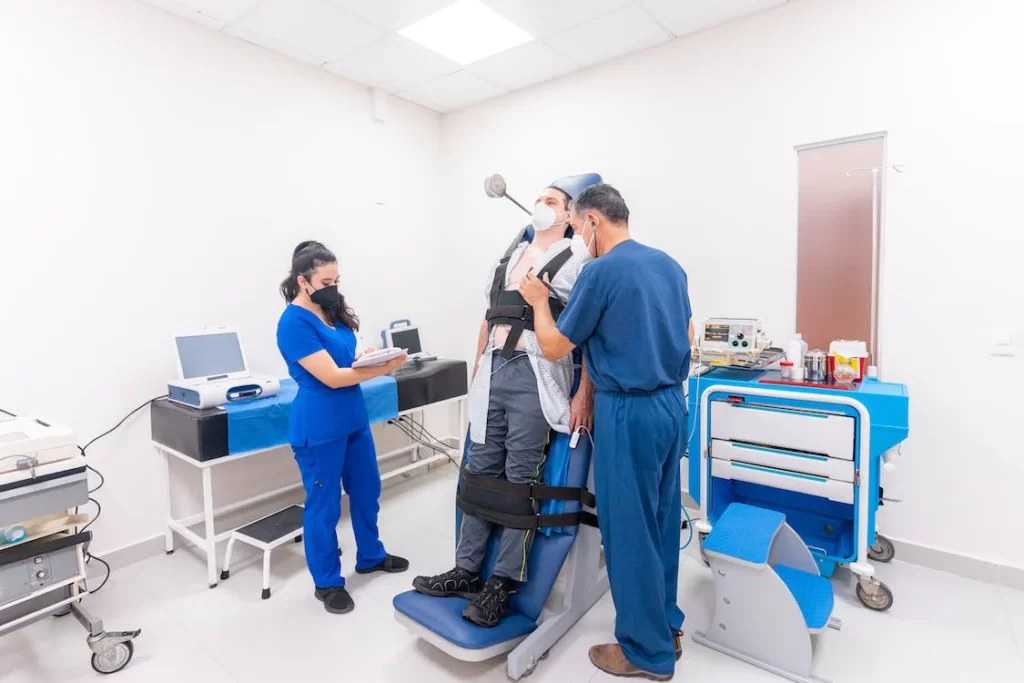
The results of a clinical study of Synchrony Medical’s LibAirty™ Airway Clearance System showed that the system was twice as effective in clearing mucus from the lungs of patients with chronic lung disease when compared to the standard of care therapy. The findings were announced in an oral presentation at CHEST2023 in Honolulu, Hawaii.
The study, led by Dr. Patrick A. Flume at the Medical University of South Carolina (MUSC), randomized patients to receive two treatments: LibAirty™ and the standard of care in random order. Additionally, participants filled out a satisfaction questionnaire, which reported higher satisfaction scores for the LibAirty™ system.
“This study showed a superior performance by the LibAirty™ system when compared to the main device used today in the US. The system was effective and well tolerated by all subjects, which suggests its potential to enhance clinical outcomes in airway clearance for patients with chronic respiratory disease,” said Dr. Flume.
The results support the findings of a prior study conducted at Sheba Medical Center, which also showed that the LibAirty™ system was more effective than other available systems and equally as effective as treatment by an experienced chest physiotherapist.
“Synchrony’s vision is to improve respiratory therapy for patients living with chronic lung disease by bringing best practice respiratory therapy to their homes. This successful trial paves the way towards marketing clearance and the commercial launch of the LibAirty™ system,” said Synchrony Medical CEO Anat Shani.
Daily airway clearance is essential for millions of patients living with chronic lung disease to prevent respiratory infections and costly hospitalizations. An effective home use device is the best solution for these patients, given the significant shortage in chest physiotherapists.